dupilumab conjunctivitis treatment
The conjunctivitis occurred at a mean of 158 weeks of treatment range 8-41 weeks and was considered severe or moderate-to-severe in 4 patients. The man received treatment with an unspecified antihistamine and corticosteroid eye drops but conjunctivitis persisted.

Dupilumab Associated Ocular Surface Disease Incidence Management And Long Term Sequelae Medrxiv
The present paper reviews the data of dupilumab-associated conjunctivitis and risk factors in adults with moderate-to-severe AD and other atopic diseases in dupilumab clinical trials and addresses the characteristics and treatment options available for.

. Mild conjunctivitis is managed with lubricating eye drops. One in 15 patients who start dupilumab treatment may develop conjunctivitis during the first 6 months most of which is manageable with ophthalmic treatments results from large study of US. In PED-OLE 12275 adolescents 44 reported one or more conjunctivitis event.
All received a prescription of trehalosehyaluroate tear substitute to hydrate conjunctival mucosa. This case was remarkable as the medication applied externally to the eyelid skin was effective in treating the conjunctival involvement possibly due to penetration of pimecrolimus through the eyelid layers. Most conjunctivitis events were mild to moderate.
8 Volume 43 Issue 8. S The main treatment for dupilumabrelated keratoconjunctivitis is topical steroids. However he developed conjunctivitis in both the eyes.
Dupilumab targets interleukin IL-4. 1 While considered a safe long-term medication for the treatment of these conditions dupilumab has been associated with conjunctivitis of varying severity. Patients on dupilumab should be advised to use these from the onset of treatment to prevent ocular symptoms.
When to Progress to Systemic Therapy. At referral he had mild erythema and lichenification of the trunk neck and extremities. Conjunctivitis a predisposition to keratoconus and anterior subcapsular cataracts are considered minor criteria of AD.
Further studies are needed to support the use of this drug for dupilumab-related conjunctivitis. Treatment options more frequently prescribed were eye drops of corticosteroids antibiotics antihistamines or mast cell stabilizers. The present review highlights the clinical presentation of dupilumab-associated conjunctivitis and addresses pharmacological and non-pharmacological options available for the treatment of this clinically highly relevant condition.
In one study topical steroids alone were sufficient in reducing signs and symptoms of acute conjunctivitis in 89 of patients. Placebo 44 recoveredresolved during the treatment period. The main treatment for dupilumabrelated keratoconjunctivitis is topical steroids.
Most conjunctivitis events were mild to moderate. Treatment options include topical corticosteroids and topical calcineurin inhibitors. In PED-OLE 12275 adolescents 44 reported one or more conjunctivitis event.
Dupilumab has a known association with ocular surface disruption and conjunctivitis although most cases are self-limiting and can be treated conservatively with artificial tears. Referral to an ophthalmologist. Conjunctivitis characteristics in dupilumab clinical trials Conjunctivitis events were mainly mild to moderate and almost all recuperated with topical eye drops until the termination of the trial period.
Some patients may require other treatment. National guidelines recommend dupilumab for the treatment of moderate to severe eczema in adults who have not improved with at least one tablet medication. Dupilumab is the first biologic approved for use in treatment of moderate to severe atopic dermatitis AD 1 2 3 4 5.
S Patients should be followed closely upon starting dupilumab and treated. The mean age at conjunctivitis onset was 30 years. Most patients with conjunctivitis dupilumab 1217.
Dupilumab may cause conjunctivitis. The risk of conjunctivitis showed no relationship with dupilumab serum concentration. About 4 years after dupilumabs approval were interested in how conjunctivitis has played out in our daily clinical practice lead study investigator Maria C.
Placebo 44 recoveredresolved during the treatment period. Dupilumab was stopped in 3 patients all of whom had severe conjunctivitis. Dermatology Times Dermatology Times August 2022 Vol.
Dupilumab binds to the alpha-chain of the interleukin IL-4 receptor thus inhibiting signaling of IL-4 and IL-13. Eric Simpson MD MCR and professor of dermatology at Oregon Health Science University presents exciting new systemic therapy treatment options for atopic dermatitis. Investigators identified 20 cases of.
Most patients with conjunctivitis dupilumab 1217. Up to 1 in 5 people taking dupilumab develop conjunctivitis which can cause itching redness and dryness of the eye. Treatments included antifungal corticosteroid cream itraconazole tablets and tacrolimus 01 ointment.
7 Close monitoring for well-known complications of concurrent steroid use including increased IOP and cataract should be put in place. At baseline 9 75 of the 12 patients had severe AD. Glaucoma cataracts posterior subscapular and and infectious keratoconjunctivitis may complicate therapy with corticosteroids.
Following three months of the dupilumab his skin symptoms improved substantially. Topical tacrolimus eyelid oin tment. The risk of conjunctivitis showed no relationship with dupilumab serum concentration.

Clinical Improvement Of Patients After 16 Weeks Of Dupilumab Treatment Download Scientific Diagram

Dupilumab An Opportunity To Unravel In Vivo Actions Of Il 4 And Il 13 In Humans Sciencedirect
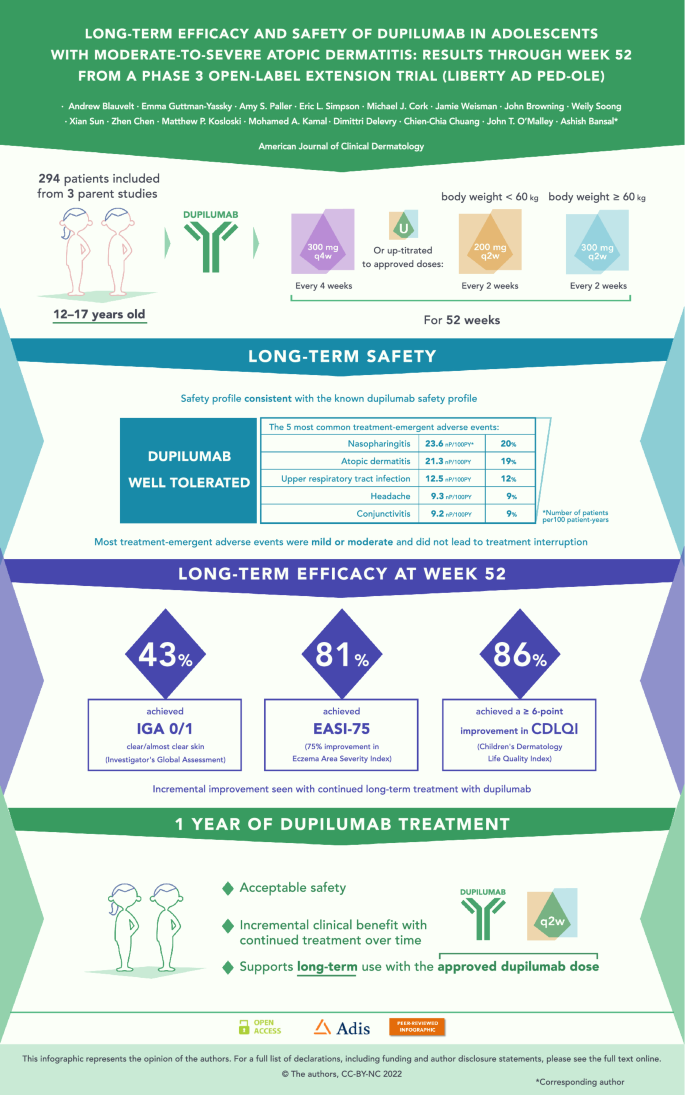
Long Term Efficacy And Safety Of Dupilumab In Adolescents With Moderate To Severe Atopic Dermatitis Results Through Week 52 From A Phase Iii Open Label Extension Trial Liberty Ad Ped Ole Springerlink
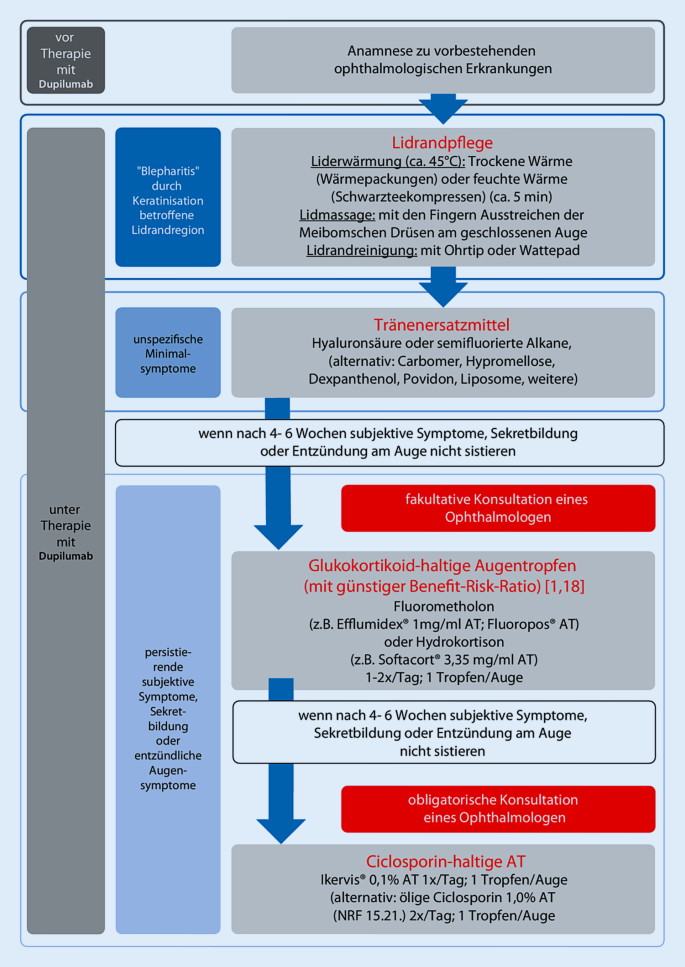
Dupilumab Zur Behandlung Der Therapierefraktaren Atopischen Dermatitis Springerlink

Real World Persistence With Dupilumab Among Adults With Atopic Dermatitis Annals Of Allergy Asthma Immunology
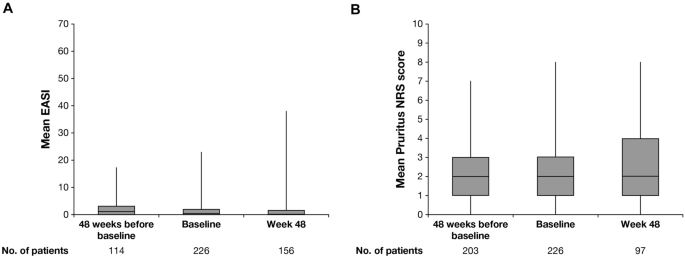
Dupilumab Provides Acceptable Safety And Sustained Efficacy For Up To 4 Years In An Open Label Study Of Adults With Moderate To Severe Atopic Dermatitis Springerlink

Dupilumab Associated Ocular Surface Disease Incidence Management And Long Term Sequelae Medrxiv

Dupilumab Senkt Den Juckreiz Und Hebt Die Lebensqualitat Rosenfluh Ch

Moderate Conjunctivitis And Blepharitis At Week 22 Of Dupilumab Treatment Download Scientific Diagram

Example Of Case Before And After Treatment With Dupilumab 300mg Every Download Scientific Diagram

Transient Increase In Circulating Basophils And Eosinophils In Dupilumab Associated Conjunctivitis In Patients With Atopic Dermatitis Html Acta Dermato Venereologica

New Insights On Ocular Surface Disease In Patients With Atopic Dermatitis Treated With Dupilumab Bortoluzzi 2022 British Journal Of Dermatology Wiley Online Library

Effects Of Dupilumab On Lesional Ad Skin A Schematic Representation Download Scientific Diagram
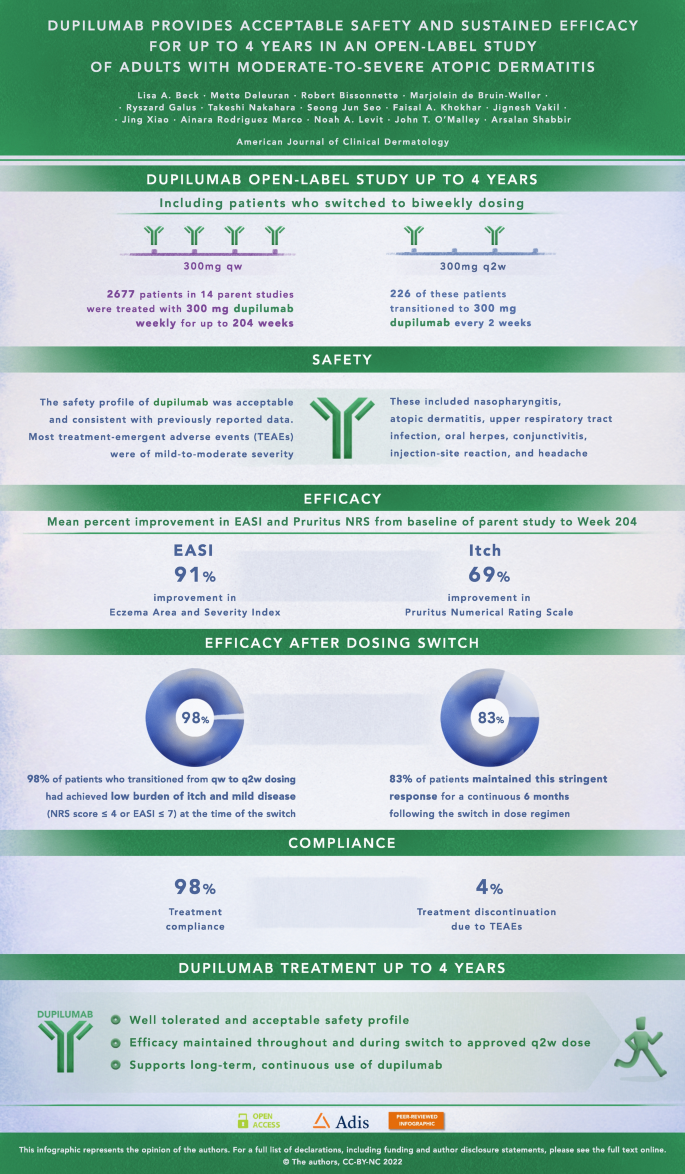
Dupilumab Provides Acceptable Safety And Sustained Efficacy For Up To 4 Years In An Open Label Study Of Adults With Moderate To Severe Atopic Dermatitis Springerlink

Dupilumab And The Risk Of Conjunctivitis And Serious Infection In Patients With Atopic Dermatitis A Propensity Score Matched Cohort Study Journal Of The American Academy Of Dermatology
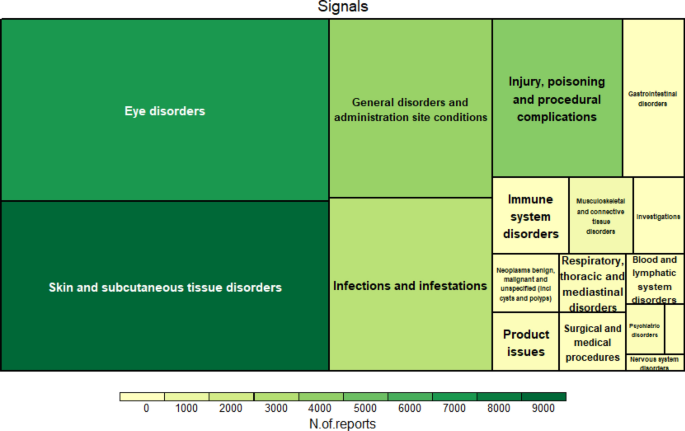
Ocular Surface Disorders Associated With The Use Of Dupilumab Based On Who Vigibase Scientific Reports

Long Term Follow Up And Treatment Outcomes Of Conjunctivitis During Dupilumab Treatment In Patients With Moderate To Severe Atopic Dermatitis The Journal Of Allergy And Clinical Immunology In Practice

Clinical Findings Of Bilateral Blepharitis And Conjunctivitis After Download Scientific Diagram

Comments
Post a Comment